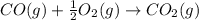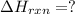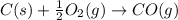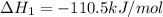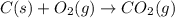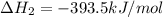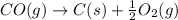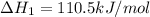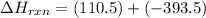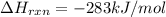CO(g) + 12 O2(g) → CO2(g)The combustion of carbon monoxide is represented by the equation above.(a) Determine the value of the standard enthalpy change, ∆HrxnD , for the combustion of CO(g) at 298 Kusing the following information. C(s) + 12 O2(g) → CO(g) ∆H298D = − 110.5 kJ mol−1 C(s) + O2(g) → CO2(g) ∆H298D = − 393.5 kJ mol−1

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, medlinalex
Compare and contrast physical changes with chemical changes.
Answers: 1


Chemistry, 22.06.2019 12:30, meghan2529
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 23.06.2019 09:40, 23rwilliamson
Is cutting your nails a physical or chemical change
Answers: 2
You know the right answer?
CO(g) + 12 O2(g) → CO2(g)The combustion of carbon monoxide is represented by the equation above.(a)...
Questions in other subjects:

English, 06.10.2019 01:10

Mathematics, 06.10.2019 01:10



History, 06.10.2019 01:10




Biology, 06.10.2019 01:10

 will be,
will be,