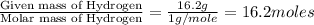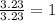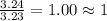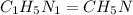Chemistry, 11.02.2020 17:21 olivernolasco23
A laboratory analysis of a sample finds it is composed of 38.8% carbon, 16.2% hydrogen, and 45.1% nitrogen. What is its empirical formula? Give your answer in the form C#H#N#, where the number following the element’s symbol corresponds to the subscript in the formula.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, tiffanyrhoda
Which of the following can be used to measure electricity
Answers: 1

Chemistry, 22.06.2019 02:30, caeyanij
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3


Chemistry, 22.06.2019 13:30, princessroseee769
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1
You know the right answer?
A laboratory analysis of a sample finds it is composed of 38.8% carbon, 16.2% hydrogen, and 45.1% ni...
Questions in other subjects:

Mathematics, 02.07.2019 16:30

Spanish, 02.07.2019 16:30




Social Studies, 02.07.2019 16:30