Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, nijanicole164
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 20:00, batoolishak7475
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
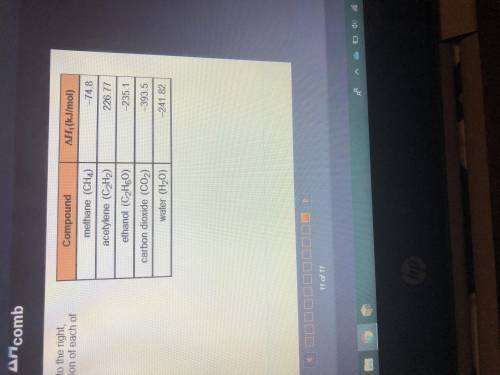
Using the information in the table to the right, calculate the enthalpy of combustion of each of the...
Questions in other subjects:


Mathematics, 14.01.2022 04:40


English, 14.01.2022 04:40

Mathematics, 14.01.2022 04:40


Mathematics, 14.01.2022 04:40

Mathematics, 14.01.2022 04:40