
Chemistry, 11.02.2020 06:00 mariahdelossantos031
Calculate the energy required to produce 7.00 mol Cl2O7 on the basis of the following balanced equation. 2Cl2(g) + 7O2(g) + 130 kcal --> 2Cl2O7(g) Select one: a. 7.00 kcal b. 65 kcal c. 130 kcal d. 455 kcal

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, mgavyn1052
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1


Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2
You know the right answer?
Calculate the energy required to produce 7.00 mol Cl2O7 on the basis of the following balanced equat...
Questions in other subjects:



Mathematics, 30.11.2021 05:50

SAT, 30.11.2021 05:50



Mathematics, 30.11.2021 05:50


English, 30.11.2021 05:50

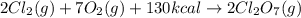


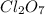 on the basis of given reaction is 455 kcal.
on the basis of given reaction is 455 kcal.

