Chemistry, 11.02.2020 05:24 alexmodersks3055
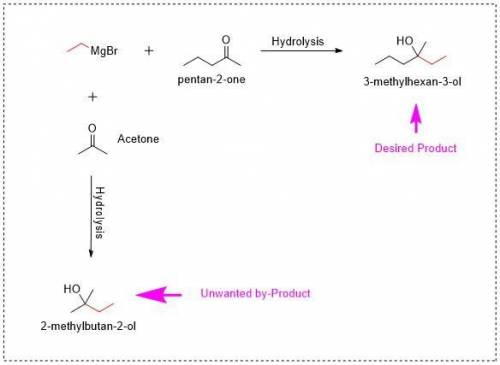
A Grignard reagent and a ketone are reacted in ether solution and, followed by an acid workup, form a tertiary alcohol. Recall that Grignard reactions must be scrupulously dry in order to work effectively. A common method of drying glassware is to rinse with acetone prior to use.
1. Why is rinsing with acetone not suitable for the reaction stated in the question.
a) Magnesium does not dissolve in acetone.
b) Water dissolves in acetone. Adding acetone will add water to the reaction flask.
c) Magnesium dissolves in acetone. Adding acetone will remove a vital reactant from the flask.
d) Acetone is a ketone. Grignard reagents will react with acetone to make an unwanted byproduct.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, zionlopez543
Americium-241 undergoes fission to produce three neutrons per fission event. if a neutron-absorbing material is mixed in with this sample so that the rate of neutron production drops down to 1.8 neutrons per fission event, which will be effective at achieving a critical mass? check all that apply. remove a deflective shield surrounding the sample. remove absorbent material mixed in with the sample. compress the sample of americium-241.
Answers: 1

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3

Chemistry, 22.06.2019 13:30, richardwalker8ourhg2
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a. the mitochondria b. the nucleus c. the vacuoles d. the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
A Grignard reagent and a ketone are reacted in ether solution and, followed by an acid workup, form...
Questions in other subjects:


History, 02.07.2019 20:40

Social Studies, 02.07.2019 20:40

History, 02.07.2019 20:40



Mathematics, 02.07.2019 20:40

Mathematics, 02.07.2019 20:40

Mathematics, 02.07.2019 20:40

Biology, 02.07.2019 20:40