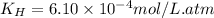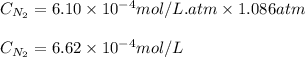Chemistry, 11.02.2020 05:05 humblemalak
The solubility of nitrogen, N2, in water is 4.52 ✕ 10−4 mol/L at 0°C when the nitrogen pressure above water is 0.741 atm. Calculate the solubility of nitrogen in water when the partial pressure of nitrogen above water is 1.086 atm at 0°C?

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1

Chemistry, 22.06.2019 17:00, BREBRE8932
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
The solubility of nitrogen, N2, in water is 4.52 ✕ 10−4 mol/L at 0°C when the nitrogen pressure abov...
Questions in other subjects:




Mathematics, 24.09.2020 14:01






Mathematics, 24.09.2020 14:01

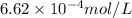

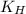 = Henry's constant = ?
= Henry's constant = ?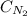 = molar solubility of nitrogen gas =
= molar solubility of nitrogen gas = 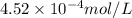
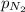 = partial pressure of nitrogen gas = 0.741 atm
= partial pressure of nitrogen gas = 0.741 atm