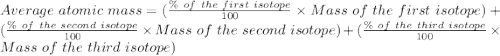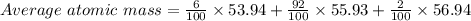Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, aedmund1225
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 21.06.2019 23:20, anggar20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

You know the right answer?
Iron (Fe) has three isotopes. Calculate the average atomic mass of the element Fe using the followin...
Questions in other subjects:




History, 12.02.2021 18:10




Mathematics, 12.02.2021 18:10

Chemistry, 12.02.2021 18:10