
Chemistry, 11.02.2020 04:24 jholland03
How much energy (heat) is required to convert 248 g of water from 0oC to 154oC? Assume that the water begins as a liquid, that the specific heat of water is 4.184 J/g. oC over the entire liquid range, that the specific heat of steam is 1.99 J/g. oC, and the heat of vaporization of water is 40.79 kJ/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:40, caleb19moody
21. consider the following chemical reaction: n2+ o2 2 no if 10.0 g of n2 reacts with excess oxygen then how many grams of no can be formed? a) 10.7 g b) 21.4 g c) 32.9 g d) 42.8 g page 4 of 8
Answers: 2


Chemistry, 23.06.2019 02:00, Turtlelover05
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3

Chemistry, 23.06.2019 03:00, KindaSmartPersonn
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
How much energy (heat) is required to convert 248 g of water from 0oC to 154oC? Assume that the wate...
Questions in other subjects:






Mathematics, 10.04.2020 21:50



Mathematics, 10.04.2020 21:50

Social Studies, 10.04.2020 21:50

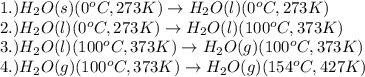
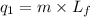
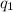 = amount of heat absorbed = ?
= amount of heat absorbed = ?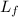 = latent heat of fusion = 334 J/g
= latent heat of fusion = 334 J/g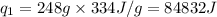
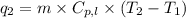
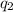 = amount of heat absorbed = ?
= amount of heat absorbed = ?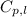 = specific heat of water = 4.184 J/g°C
= specific heat of water = 4.184 J/g°C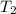 = final temperature =
= final temperature = 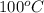
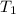 = initial temperature =
= initial temperature = 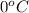
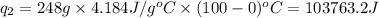
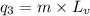
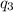 = amount of heat absorbed = ?
= amount of heat absorbed = ?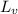 = latent heat of vaporization =
= latent heat of vaporization = 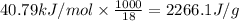 (Conversion factor used: 1 kJ = 1000 J and molar mass of water = 18 g/mol)
(Conversion factor used: 1 kJ = 1000 J and molar mass of water = 18 g/mol)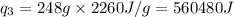
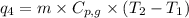
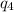 = amount of heat absorbed = ?
= amount of heat absorbed = ?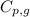 = specific heat of steam = 1.99 J/g°C
= specific heat of steam = 1.99 J/g°C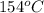
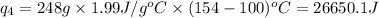
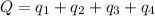
![Q=[84832+103763.2+560480+26650.1]J=775,725.3J=775.7kJ](/tpl/images/0506/1413/615e7.png)


