
Chemistry, 11.02.2020 04:25 Andychulo7809
Calculate the amount of heat (in kJ) required to convert 62.6 g of water to steam at 100°C. (The molar heat of vaporization of water is 40.79 kJ/mol.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:50, psychocatgirl1
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3


Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
You know the right answer?
Calculate the amount of heat (in kJ) required to convert 62.6 g of water to steam at 100°C. (The mol...
Questions in other subjects:



Arts, 23.07.2019 13:30

Health, 23.07.2019 13:30

Physics, 23.07.2019 13:30


English, 23.07.2019 13:30


Advanced Placement (AP), 23.07.2019 13:30

Arts, 23.07.2019 13:30

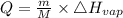
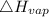 = 40.79 kJ/mol
= 40.79 kJ/mol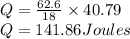
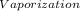 ·m where Q = required heat energy,
·m where Q = required heat energy, 

