Dinitrogen tetraoxide is a colorless gas at room temperature. It can dissociate into nitrogen dioxide, which is a reddish brown gas.
N2O4(g) <> 2 NO2(g)
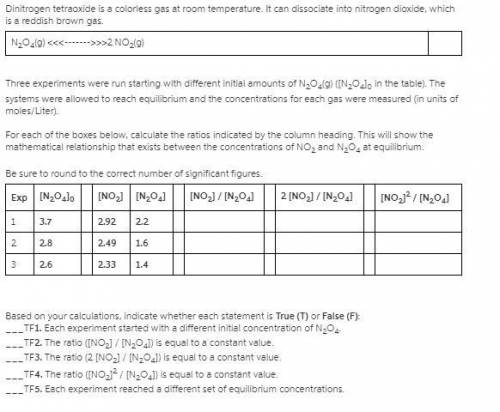
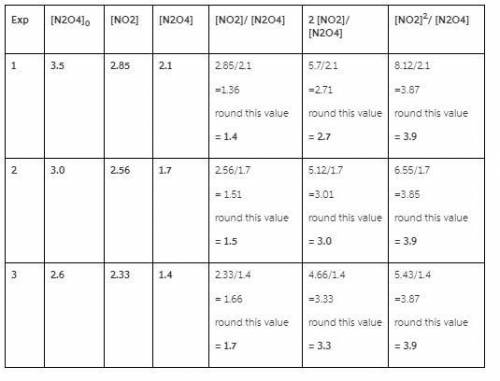
Three experiments were run starting with different initial amounts of N2O4(g) ([N2O4]0 in the table). The systems were allowed to reach equilibrium and the concentrations for each gas were measured (in units of moles/Liter).
1. For each of below, calculate the ratios indicated by the column heading. This will show the mathematical relationship that exists between the concentrations of NO2 and N2O4 at equilibrium.
Be sure to round to the correct number of significant figures.
Exp [N2O4]0 [NO2] [N2O4] [NO2] / [N2O4] 2 [NO2] / [N2O4] [NO2]2 / [N2O4]
1 3.7 2.92 2.2
2 3.0 2.56 1.7
3 2.1 2.06 1.1
2. Based on your calculations, indicate whether each statement is True (T) or False (F):
1. Each experiment started with a different initial concentration of N2O4.
2. The ratio ([NO2] / [N2O4]) is equal to a constant value.
3. The ratio (2 [NO2] / [N2O4]) is equal to a constant value.
4. The ratio ([NO2]2 / [N2O4]) is equal to a constant value.
5. Each experiment reached a different set of equilibrium concentrations.

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, bibhu42kumarp7o4ss
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 13:50, kelonmazon2492
Read the chemical equation. 2c2h2 + 5o2 → 4co2 + 2h2o which of the following statements would be correct if one mole of c2h2 was used in this reaction? one mole of oxygen was used in this reaction. five moles of oxygen were used in this reaction. four moles of carbon dioxide were produced from this reaction. two moles of carbon dioxide were produced from this reaction.
Answers: 3

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
Dinitrogen tetraoxide is a colorless gas at room temperature. It can dissociate into nitrogen dioxid...
Questions in other subjects:








History, 22.01.2020 04:31


Mathematics, 22.01.2020 04:31

![[NO2]^{2}/ [N2O4]](/tpl/images/0505/8775/308c2.png) in all three experiment have same value 3.9, 3.9 & 3.9 is a constant value.
in all three experiment have same value 3.9, 3.9 & 3.9 is a constant value.