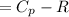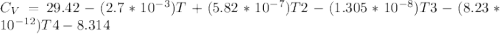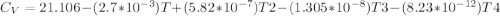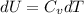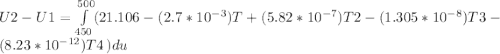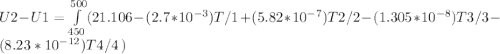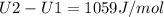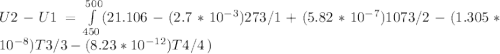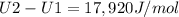The ideal gas heat capacity of nitrogen varies with temperature. It is given by:
Cp = 29.42 -...

Chemistry, 10.02.2020 23:04 steventhecool22
The ideal gas heat capacity of nitrogen varies with temperature. It is given by:
Cp = 29.42 - (2.170*10^-3 ) T + (0.0582*10^-5 ) T2 + (1.305*10^-8 ) T3 – (0.823*10^-11) T4
T in K and Cp in Joule/(mole-K). Assuming that N2 is an ideal gas:
A) How much internal energy (per mole) must be added to nitrogen to increase its temperature from 450 to 500 K. B) Repeat part A for an initial temperature of 273 K and final temperature of 1073 K.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 07:50, dootdootkazoot
What is the significance sodium hydroxide and hydrochloric acid
Answers: 1

Chemistry, 23.06.2019 08:00, kendrawalraven
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 3
You know the right answer?
Questions in other subjects:

Biology, 05.10.2019 08:30

Mathematics, 05.10.2019 08:30


Mathematics, 05.10.2019 08:30






Chemistry, 05.10.2019 08:30

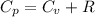
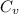 from above if we make
from above if we make