Chemistry, 10.02.2020 23:04 natalie2sheffield
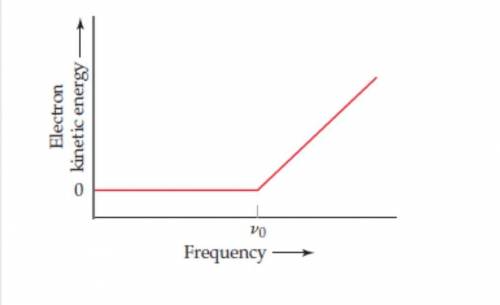
In an experiment to study the photoelectric effect, a scientist measures the kinetic energy of ejected electrons as afunction of the frequency of radiation hitting a metal surface. She obtains the following plot The point labeled " v0 "corresponds to light with a wavelength of 680 nrn (a)What is the value of in 5-1? (b)What is the value of the work functionof the metal in units of of ki/mol ejected electrons? (c) What happens when the metal is irradiated with light of frequencyless than Vo? (d) Note that when the frequency of the light is greater than Vo, the plot shows a straight line with a nonzeroslope. Why is this the case? (e) Can you determine the slope of the line segment discussed in part (d)? Explain.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ciarakelly636owuiup
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1

Chemistry, 21.06.2019 22:00, breannaasmith1122
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 00:00, tahjaybenloss16
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
In an experiment to study the photoelectric effect, a scientist measures the kinetic energy of eject...
Questions in other subjects:










Mathematics, 18.07.2019 05:20