Chemistry, 10.02.2020 22:26 jaaiphieaslade
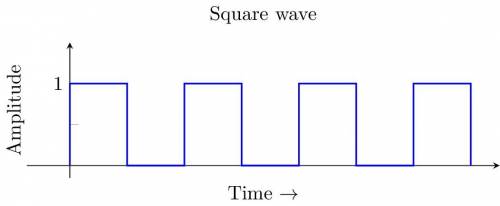
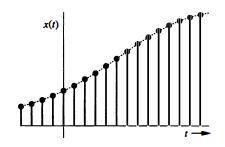
1.3-3 Determine whether each of the following statements is true or false. If the statement is false, demonstrate this by proof or example. (a) Every continuous-time signal is an analog signal. (b) Everydiscrete-. (c) If a signal is not an energy signal, then it must be a power signal and vice versa. (d) An energy signal must be of finite duration. (e) A power signal cannot be causal. (f) A periodic signal cannot be anticausal

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, toledanomariap43bxm
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
1.3-3 Determine whether each of the following statements is true or false. If the statement is false...
Questions in other subjects:


English, 17.04.2020 21:15






Biology, 17.04.2020 21:16


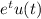 signal
signal