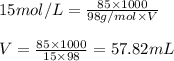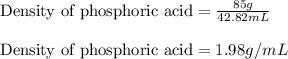Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, shadekashakay
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3


Chemistry, 22.06.2019 16:00, yfnal3x
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2
You know the right answer?
Concentrated phosphoric acid is sold as a solution of 85% phosphoric acid and 15% water by mass. Giv...
Questions in other subjects:





Social Studies, 29.05.2020 16:58

Spanish, 29.05.2020 16:58


Mathematics, 29.05.2020 16:58


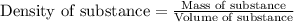 ......(1)
......(1)