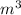A balloon is filled with 1 mole of helium gas at 100 kPa of pressure and a
temperature of 300 K...

Chemistry, 10.02.2020 21:07 cordobamariana07
A balloon is filled with 1 mole of helium gas at 100 kPa of pressure and a
temperature of 300 K. What is the approximate volume of the balloon? (The
ideal gas law and gas constant are given below.)
PV = nRT
R=8.314 m. Pa/
mol K


Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1


Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
Questions in other subjects:



Mathematics, 13.12.2021 22:30




Mathematics, 13.12.2021 22:30


Chemistry, 13.12.2021 22:30

Geography, 13.12.2021 22:30