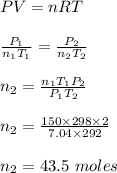Chemistry, 10.02.2020 05:39 Sonicawesomeness
A steel cylinder contains 150.0 mol argon gas at a temperature of 25°C and a pressure of 7.04 MPa. After some argon has been used, the pressure is 2.00 MPa at a temperature of 19°C. What mass (g) of argon remains in the cylinder?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, tamiaollie
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2

Chemistry, 21.06.2019 19:00, 21brooklynmartin
Does the temperature affect the solubility of sugar and salt in water? if it does tell me like different temperatures with different solubilities so i can sketch down a graph
Answers: 2

You know the right answer?
A steel cylinder contains 150.0 mol argon gas at a temperature of 25°C and a pressure of 7.04 MPa. A...
Questions in other subjects:



Mathematics, 22.04.2021 20:30

Chemistry, 22.04.2021 20:30


Mathematics, 22.04.2021 20:30

Mathematics, 22.04.2021 20:30


History, 22.04.2021 20:30