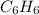Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 22.06.2019 17:50, mytymikey123
You exhale co2 which is produced during cellular respiration. co2 combines with the water in your blood's plasma to make up one half of the body's most important buffer pair, carbonic acid. the more physical activity you engage in, the more co2 your body is producing. you can see this by putting some of the cabbage indicator in a glass and then blowing bubbles into it through a straw. can you see a change in the color of the indicator?
Answers: 2
You know the right answer?
Determine the molecular formula of the compound with an empirical formula of ch and a molar mass of...
Questions in other subjects:

English, 29.10.2020 04:50


Mathematics, 29.10.2020 04:50

Mathematics, 29.10.2020 04:50



History, 29.10.2020 04:50

Health, 29.10.2020 04:50


Health, 29.10.2020 04:50