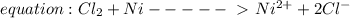cl2 + ni = ni2+ + 2cl-

Chemistry, 04.10.2019 18:00 monster5368
What is the overall cell potential for this redox reaction?
cl2 + ni = ni2+ + 2cl-
cl2 + 2e- = 2cl- 1.36 v
ni = ni2+ + 2e- -0.25 v
0.62 v
b.)0.61 v
c.)1.01 v
d.)1.61 v
e.)2.06 v

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 12:30, azzyla2003
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1
You know the right answer?
What is the overall cell potential for this redox reaction?
cl2 + ni = ni2+ + 2cl-
cl2 + ni = ni2+ + 2cl-
Questions in other subjects:


Mathematics, 24.10.2020 06:30


Mathematics, 24.10.2020 06:30

Mathematics, 24.10.2020 06:30


English, 24.10.2020 06:30

History, 24.10.2020 06:30