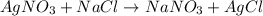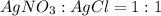Chemistry, 18.09.2019 23:50 cfnewton09
The reaction below shows how silver chloride can be synthesized.
agno3 + nacl mc017-1.jpg nano3 + agcl
how many moles of silver chloride are produced from 15.0 mol of silver nitrate?
1) 1.0 mol
2) 15.0 mol
3) 30.0 mol
4) 45.0 mol

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, emilyborland50
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d. the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 22.06.2019 04:30, terrancebest
Which is a chemical property of iron? a. it forms iron oxide (rust) when exposed to moisture and air. b. it is a gray–black metal that is hard to the touch. c. it has a melting point of 2795°f (1536°c). d. it is a good conductor of heat
Answers: 2

Chemistry, 22.06.2019 16:50, brandiwingard
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
The reaction below shows how silver chloride can be synthesized.
agno3 + nacl mc017-1.jpg nano...
agno3 + nacl mc017-1.jpg nano...
Questions in other subjects:

Chemistry, 24.10.2021 01:00

Biology, 24.10.2021 01:00




Geography, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00

Chemistry, 24.10.2021 01:00

Mathematics, 24.10.2021 01:00

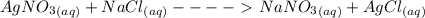
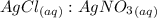 = 1 : 1
= 1 : 1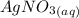 = 15.0 mol
= 15.0 mol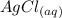 = 15.0 mol
= 15.0 mol