Chemistry, 04.02.2020 05:51 elijahjacksonrp6z2o7
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k to liquify the sample and lower the temperature to 335.0 k? the following physical data may be useful.
hvap = 33.9 kj/molhfus = 9.8 kj/mol
cliq = 1.73 j/g

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 03:00, parisaidan366
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 06:00, rigobertogarza2
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2
You know the right answer?
How much energy must be removed from a 125 g sample of benzene (molar mass= 78.11 g/mol) at 425.0 k...
Questions in other subjects:

Mathematics, 30.06.2019 15:50

English, 30.06.2019 15:50

Social Studies, 30.06.2019 15:50

Spanish, 30.06.2019 15:50


Social Studies, 30.06.2019 15:50

Physics, 30.06.2019 15:50

History, 30.06.2019 15:50

Mathematics, 30.06.2019 15:50

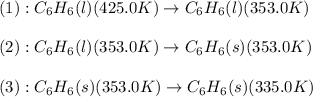
![\Delta H=[m\times c_{p,l}\times (T_{final}-T_{initial})]+m\times \Delta H_{fusion}+[m\times c_{p,s}\times (T_{final}-T_{initial})]](/tpl/images/0499/2996/53889.png)
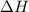 = heat available for the reaction =
= heat available for the reaction = 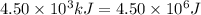
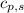 = specific heat of solid benzene =
= specific heat of solid benzene = 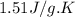
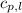 = specific heat of liquid benzene =
= specific heat of liquid benzene = 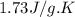
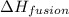 = enthalpy change for fusion =
= enthalpy change for fusion = 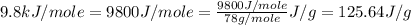
![\Delta H=[125g\times 1.73J/g.K\times (353-425)K]+125g\times -125.64J/g+[125g\times 1.51J/g.K\times (335-353)K]](/tpl/images/0499/2996/d60b8.png)