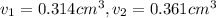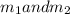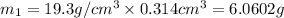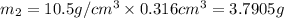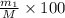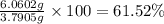Chemistry, 11.11.2019 06:31 kimloveswim
Gold is alloyed(mixed) with other metals to increase its hardness in making jewelry. (a) consider a piece of gold jewelry that weighs 9.85 g and has a volume of 0.675 cm3. the jewelry contains only gold and silver, which have densities of 19.3 g/cm3 and 10.5 g/cm3, repectively. if the total volume of the jewelry is the sum of the volumes of the gold and silver that it contains. calculate the percentage of gold(by mass) in the jewelry. (b) the relative amount of gold in an alloy is commonly expressed in units of karats.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 10:00, sdlesley66
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1


Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2
You know the right answer?
Gold is alloyed(mixed) with other metals to increase its hardness in making jewelry. (a) consider a...
Questions in other subjects:




Mathematics, 18.03.2021 02:10




Mathematics, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10

Mathematics, 18.03.2021 02:10

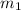
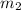
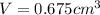
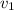
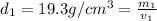
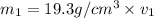
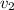
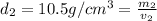
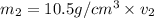
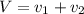
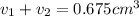 ..(1)
..(1)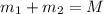
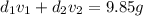
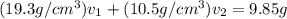 ..(2)
..(2)