
Chemistry, 29.01.2020 05:40 herringalyssa
What volume of hydrogen gas is required to react with 113 liters of ethylene (c2h4) according to the following reaction? (all gases are at the same temperature and pressure.) hydrogen (g) + ethylene (c2h4) (g) ethane (c2h6) (g)

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2



Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1
You know the right answer?
What volume of hydrogen gas is required to react with 113 liters of ethylene (c2h4) according to the...
Questions in other subjects:










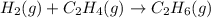
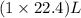 of ethylene reacts with
of ethylene reacts with 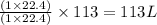 of hydrogen gas
of hydrogen gas


