Consider this reaction:
2cl2o5 (g) → 2cl2 (g) + 5o2 (g)
at a certain temp...

Consider this reaction:
2cl2o5 (g) → 2cl2 (g) + 5o2 (g)
at a certain temperature it obeys this rate law.
rate = (6.48 m-1 • s-1)[cl2o5]2
suppose a vessel contains cl2o5 at a concentration of 1.16 m. calculate the concentration of cl2o5 in the vessel 0.820 seconds later. you may assume no other reaction is important.
round your answer to 2 significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, steven2996
What can be the use of smoke transformed into liquid?
Answers: 1

Chemistry, 21.06.2019 14:30, twinkieslayer
As a part of an experiment a student burns propane to produce carbon dioxide and water she remembers that she must follow the law conservation of matter when writing a balanced chemical equation which of these equation adheres to the law of conservation of matter
Answers: 1

Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 15:20, Tringirl233
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3
You know the right answer?
Questions in other subjects:






History, 18.07.2020 22:01


Mathematics, 18.07.2020 22:01


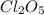 in the vessel 0.820 seconds later is, 0.16 M
in the vessel 0.820 seconds later is, 0.16 M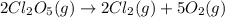
![rate=(6.48M^{-1}s^{-1})[Cl_2O_5]^2](/tpl/images/0479/5701/ef798.png)
![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0479/5701/ccade.png)
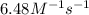
![[A_t]](/tpl/images/0479/5701/5262c.png) = final concentration = ?
= final concentration = ?![[A_o]](/tpl/images/0479/5701/dc622.png) = initial concentration = 1.16 M
= initial concentration = 1.16 M![6.48\times 0.820=\frac{1}{[A_t]}-\frac{1}{1.16}](/tpl/images/0479/5701/3da76.png)
![[A_t]=0.16M](/tpl/images/0479/5701/3ee9b.png)


