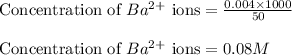Chemistry, 29.01.2020 00:48 brooke3493
A20.0 ml sample of 0.200 m k₂co₃ solution is added to 30.0 ml of 0.400 m ba(no₃)₂ solution. barium carbonate precipitates.
the concentration of barium ion, ba²⁺, in solution after the reaction is

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, jbarbie3
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 22.06.2019 18:00, ameliaxbowen7
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
A20.0 ml sample of 0.200 m k₂co₃ solution is added to 30.0 ml of 0.400 m ba(no₃)₂ solution. barium c...
Questions in other subjects:



Biology, 29.09.2019 23:00




Mathematics, 29.09.2019 23:00

Mathematics, 29.09.2019 23:00

History, 29.09.2019 23:00

Mathematics, 29.09.2019 23:00

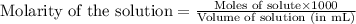 .....(1)
.....(1)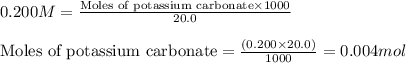
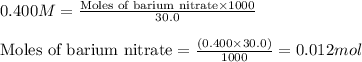
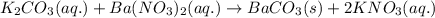
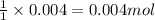 of barium nitrate
of barium nitrate