
At a certain temperature this reaction follows second-order kinetics with a rate constant of 14.1 m⁻¹s⁻¹ : > 2so₃g + 2so₂g o₂g suppose a vessel contains so₃ at a concentration of 1.44m . calculate the concentration of so₃ in the vessel 0.240 seconds later. you may assume no other reaction is important. round your answer to 2 significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2


You know the right answer?
At a certain temperature this reaction follows second-order kinetics with a rate constant of 14.1 m⁻...
Questions in other subjects:










Biology, 22.04.2020 04:01

![kt=\frac{1}{[A_t]}-\frac{1}{[A_o]}](/tpl/images/0479/1273/ccade.png)
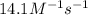
![[A_t]](/tpl/images/0479/1273/5262c.png) = final concentration = ?
= final concentration = ?![[A_o]](/tpl/images/0479/1273/dc622.png) = initial concentration = 1.44 M
= initial concentration = 1.44 M![14.1\times 0.240=\frac{1}{[A_t]}-\frac{1}{1.44}](/tpl/images/0479/1273/20832.png)
![[A_]t=0.24M](/tpl/images/0479/1273/ae6b5.png)


