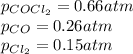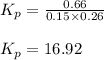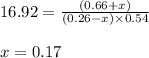Chemistry, 28.01.2020 21:48 sierravick123owr441
The system described by the reaction co(g)+cl2(g)⇌cocl2(g) is at equilibrium at a given temperature when pco= 0.26 atm , pcl2= 0.15 atm , and pcocl2= 0.66 atm . an additional pressure of cl2(g)= 0.39 atm is added. find the pressure of co when the system returns to equilibrium.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 07:30, candigirl8847
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1


Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
The system described by the reaction co(g)+cl2(g)⇌cocl2(g) is at equilibrium at a given temperature...
Questions in other subjects:







Spanish, 28.02.2021 17:00



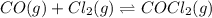
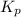 for above equation follows:
for above equation follows: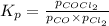 .......(1)
.......(1)