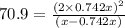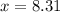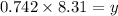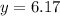Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1

Chemistry, 22.06.2019 20:30, ashley4329
Select all the correct answers. which compounds have the empirical formula ch20? (multiple answers)a. c2h4o2b. c3h603c. ch2o2d. c5h1005e. c6h1206
Answers: 2
You know the right answer?
At particular temperature, kp = 70.9 for the following reactionn2o4(g) == 2no2(g)1. a certain pressu...
Questions in other subjects:



Physics, 04.08.2019 13:50

World Languages, 04.08.2019 13:50

History, 04.08.2019 13:50

History, 04.08.2019 14:00


English, 04.08.2019 14:00


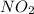 is, 12.34 atm
is, 12.34 atm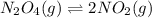
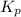 for above reaction follows:
for above reaction follows: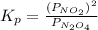 ........(1)
........(1)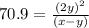 ..............(2)
..............(2)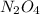 remains at equilibrium. That means,
remains at equilibrium. That means,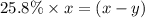
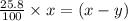
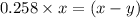
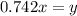 ..............(3)
..............(3)