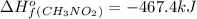Top fuel dragsters and funny cars burn nitro-methane as fuel according to the following balanced combustion equation: 2ch3no2(l)+3/2o2(g)→2co2(g)+3h2o(g) +n2(g). the standard enthalpy of combustion for nitromethane is −709.2kj/mol. calculate the standard enthalpy of formation(delta h formation) for nitro-methane.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, fvmousdiana
Determine the percent sulfuric acid by mass of a 1.61 m aqueous solution of h2so4. %
Answers: 2

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
Top fuel dragsters and funny cars burn nitro-methane as fuel according to the following balanced com...
Questions in other subjects:

Mathematics, 06.04.2021 02:00

Chemistry, 06.04.2021 02:00



Biology, 06.04.2021 02:00




Mathematics, 06.04.2021 02:00

English, 06.04.2021 02:00

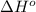
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0475/2966/45485.png)
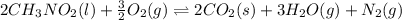
![\Delta H^o_{rxn}=[(n_{(CO_2)}\times \Delta H^o_f_{(CO_2)})+(n_{(H_2O)}\times \Delta H^o_f_{(H_2O)})+(n_{(N_2)}\times \Delta H^o_f_{(N_2)})]-[(n_{(CH_3NO_2)}\times \Delta H^o_f_{(CH_3NO_2)})+(n_{(O_2)}\times \Delta H^o_f_{(O_2)})]](/tpl/images/0475/2966/ea72d.png)
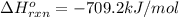
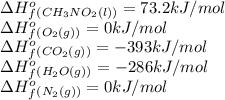
![-709.2=[(2\times -393)+(3\times -286)+(1\times 0)]-[(2\times \Delta H^o_f_{(CH_3NO_2)})+(\frac{3}{2}\times 0)]](/tpl/images/0475/2966/4d16f.png)