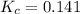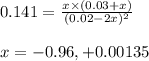Chemistry, 28.01.2020 05:31 lilacastro
At 25°c, the equilibrium constant kc for the reaction in thesolvent ccl4 2brcl < > br2 + cl2 is 0.141. if the initial concentration of chlorine is 0.0300 m andof bromine monochloride is 0.0200 m, what is the equilibrium concentration of bromine?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 14:50, jonmorton159
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 23.06.2019 01:10, minasotpen1253
Volume is a measurement of how fast particles of a substance are moving
Answers: 3
You know the right answer?
At 25°c, the equilibrium constant kc for the reaction in thesolvent ccl4 2brcl < > br2 + cl2...
Questions in other subjects:






Mathematics, 04.02.2020 16:55

Social Studies, 04.02.2020 16:55

Business, 04.02.2020 16:55

Mathematics, 04.02.2020 16:55

English, 04.02.2020 16:55

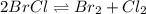
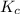 for above equation follows:
for above equation follows:![K_c=\frac{[Br_2]\times [Cl_2]}{[BrCl]^2}](/tpl/images/0475/3007/a6ccc.png)