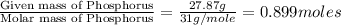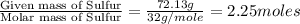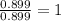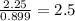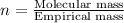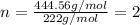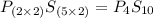Answers: 2


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:30, SavageKidKobe
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
Ferrophosphorus (fe2p) reacts with pyrite (fes2), producing iron (ii) sulfide and a compound that is...
Questions in other subjects:

Mathematics, 21.09.2021 07:10


Mathematics, 21.09.2021 07:10

Mathematics, 21.09.2021 07:10

Biology, 21.09.2021 07:10


Mathematics, 21.09.2021 07:10



Mathematics, 21.09.2021 07:10

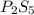 and
and 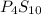 respectively.
respectively.