Chemistry, 28.01.2020 05:31 PastyMexican24
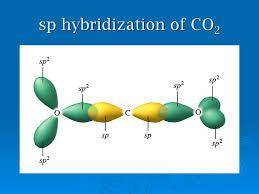
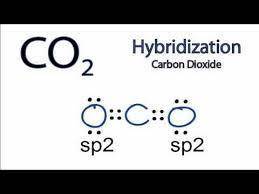
The molecule co2 has two c-o double bonds. describe the bonding in the co2 molecule. which involves hybrid orbitals for carbon and oxygen?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, ashleyjaslin
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1


Chemistry, 22.06.2019 03:00, bobbycisar1205
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 06:40, alyons60
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3
You know the right answer?
The molecule co2 has two c-o double bonds. describe the bonding in the co2 molecule. which involves...
Questions in other subjects:

History, 23.08.2019 06:00






Mathematics, 23.08.2019 06:00

Mathematics, 23.08.2019 06:00

Mathematics, 23.08.2019 06:00

Mathematics, 23.08.2019 06:00