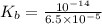Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, rscott2649
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 06:00, Kjswagout5052
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 12:00, ctyrector
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
The acid-dissociation constant, ka, for benzoic acid is 6.5 × 10-5. which will you use to calculate...
Questions in other subjects:



Advanced Placement (AP), 17.10.2021 09:40


English, 17.10.2021 09:40


Mathematics, 17.10.2021 09:40

Physics, 17.10.2021 09:40

History, 17.10.2021 09:40