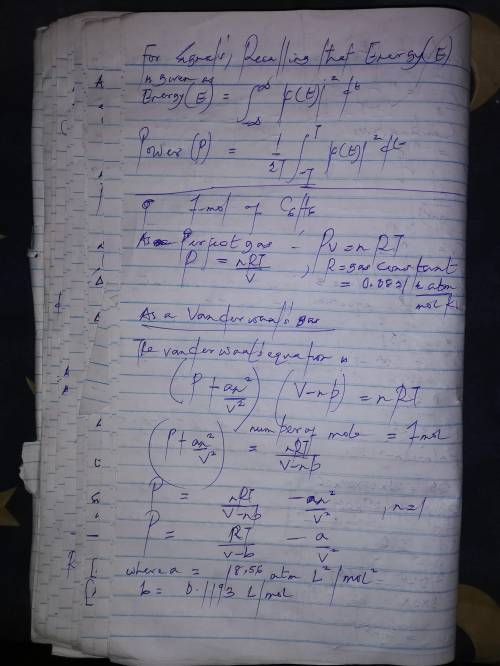Chemistry, 28.01.2020 02:31 Desinfektionsmittel
Calculate the pressure exerted by 1.0 mol of c6h6behaving as a)a perfect gas and b) a van der waals gas when it is confined under the following conditions: i)373.15 k in 22.414 dm3ii)1000 k in 22.414 dm3iii)1000 k in 150.000 dm3at which one of these conditions does the real gas be?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 01:30, kenldykido2300
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 09:00, cyni
Which question could be best answered using the process of scientific inquiry? do different plates have different rock compositions? why did it take so long to develop the theory of plate tectonics? what are different cultural myths caused by plate tectonics? do plates move intentionally to cause volcanic eruptions?
Answers: 3

Chemistry, 23.06.2019 11:30, amiechap12
Which of the following is a possible formula unit? (2 points) select one: a. pbo b. li2b c. al2pb3 d. clo
Answers: 1
You know the right answer?
Calculate the pressure exerted by 1.0 mol of c6h6behaving as a)a perfect gas and b) a van der waals...
Questions in other subjects:

Mathematics, 15.09.2021 03:30



Arts, 15.09.2021 03:30


English, 15.09.2021 03:30

Mathematics, 15.09.2021 03:30

Physics, 15.09.2021 03:30

Mathematics, 15.09.2021 03:30

Mathematics, 15.09.2021 03:30