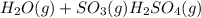Chemistry, 28.01.2020 01:31 charlesrogers38
For the endothermic formation of sulfuric acid h2o(g) + so3(g) ↔ h2so4(g) the pressure of the system and the temperature of the system would shift the reaction to the left.
1. decreasing, lowering
2. decreasing, raising
3. increasing, raising
4. increasing, lowering

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, Countryqueen525
Asa choose the correct set of reaction coefficients to properly balance the following chemical equation according to the law of conservation of mass: __s8 + __o2 ==> __so2 1, 1, 8 1, 8, 1 1, 8, 8 8, 1, 1
Answers: 1

Chemistry, 22.06.2019 11:00, Usman458
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 19:00, georgesarkes12
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
For the endothermic formation of sulfuric acid h2o(g) + so3(g) ↔ h2so4(g) the pressure of the syste...
Questions in other subjects:


Mathematics, 22.04.2021 06:20

Mathematics, 22.04.2021 06:20


Mathematics, 22.04.2021 06:20


History, 22.04.2021 06:20



History, 22.04.2021 06:20