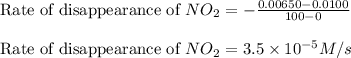Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, alanflores40
Amonoprotic acid is an acid that donates a single proton to the solution. suppose you have 0.140 g of a monoprotic acid dissolved in 35.0 ml of water. this solution is then neutralized with 14.5 ml of 0.110 m naoh. what is the molar mass of the acid?
Answers: 1

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1

Chemistry, 22.06.2019 19:30, Karinaccccc
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 00:30, runninglovexoxo
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
Nitrogen dioxide decomposes to nitric oxide and oxygen via the reaction: 2no2 → 2no + o2 in a parti...
Questions in other subjects:

English, 19.02.2020 17:04


Social Studies, 19.02.2020 17:04

History, 19.02.2020 17:04




Mathematics, 19.02.2020 17:04


 is
is 

![\text{Rate of disappearance of }NO_2=-\frac{\Delta [NO_2]}{\Delta t}](/tpl/images/0473/6156/ea698.png)
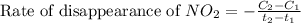
 = final concentration of
= final concentration of 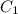 = initial concentration of
= initial concentration of 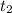 = final time = 100 minutes
= final time = 100 minutes = initial time = 0 minutes
= initial time = 0 minutes