
Suppose of zinc chloride is dissolved in of a aqueous solution of ammonium sulfate. calculate the final molarity of zinc cation in the solution. you can assume the volume of the solution doesn't change when the zinc chloride is dissolved in it. round your answer to significant digits.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, rileyallen4186pd5tgy
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1


Chemistry, 22.06.2019 19:50, jakaylathomas11
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1
You know the right answer?
Suppose of zinc chloride is dissolved in of a aqueous solution of ammonium sulfate. calculate the fi...
Questions in other subjects:

English, 05.12.2020 14:00

English, 05.12.2020 14:00



Mathematics, 05.12.2020 14:00


Business, 05.12.2020 14:00

SAT, 05.12.2020 14:00

History, 05.12.2020 14:00

English, 05.12.2020 14:00

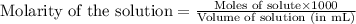 .....(1)
.....(1)
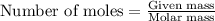


 of potassium carbonate
of potassium carbonate of acetate ion
of acetate ion


