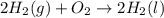Chemistry, 27.01.2020 20:31 saucyboyFredo
8. what is the heat of reaction when hydrogen and oxygen combine to form water?
2h2(g) + o2(g) → 2h2o(1)
(ahc h20(i) = -285.8 kj/mol)
a -120.9 kj
b-241.8 kj
c-571.6 kj
d-285.8 kj

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, reeceslife481
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 08:30, mosthatedpicky1
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1
You know the right answer?
8. what is the heat of reaction when hydrogen and oxygen combine to form water?
2h2(g) + o2(g...
2h2(g) + o2(g...
Questions in other subjects:

Mathematics, 12.01.2021 01:20

Mathematics, 12.01.2021 01:20

Health, 12.01.2021 01:20


Mathematics, 12.01.2021 01:20


Mathematics, 12.01.2021 01:20



Mathematics, 12.01.2021 01:20