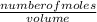Chemistry, 27.01.2020 18:31 chaitanyac90
2pb(s) + o2(aq) + 4h+(aq) → 2h2o(l) + 2pb2+(aq)
if 100 l of 0.13m lead solution is produced from the corrosion reaction, how many grams of lead metal reacted?
the answer is 0.269 g, but i don't understand the math behind getting this answer. ?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, lisbet123085
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3


Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 20:30, ShahinF7536
Some familiar products contain some of the same types of atoms. for instance, the chemical formula for baking soda is nahco 3. the chemical formula for liquid bleach is naclo, and the chemical formula for table salt is nacl. which choice best describes why these three products have some of the same types of atoms in common?
Answers: 1
You know the right answer?
2pb(s) + o2(aq) + 4h+(aq) → 2h2o(l) + 2pb2+(aq)
if 100 l of 0.13m lead solution is produced f...
if 100 l of 0.13m lead solution is produced f...
Questions in other subjects:







History, 14.07.2019 06:30

English, 14.07.2019 06:30


History, 14.07.2019 06:30