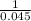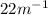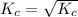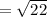Chemistry, 27.01.2020 08:31 janiyahlanae66
the reaction a(b) = 2b(g) has an equilibrium constant of k = 0.045. what is the equilibrium constant for the reaction b(g) =1/2a
express your answer using two significant figures.
i

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1



Chemistry, 23.06.2019 02:30, kieante01
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
the reaction a(b) = 2b(g) has an equilibrium constant of k = 0.045. what is the equilibrium constant...
Questions in other subjects:

Arts, 28.08.2019 15:00

Biology, 28.08.2019 15:00




Physics, 28.08.2019 15:00




Mathematics, 28.08.2019 15:00

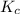 for the reaction
for the reaction 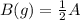 will be 4.69.
will be 4.69.![K_c=[B]^2[A]](/tpl/images/0472/2521/5d2b2.png)
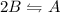
![K_c = \frac{[A]}{[B]^2}](/tpl/images/0472/2521/8045b.png)