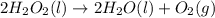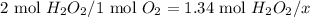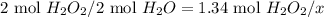Dihydrogen dioxide decomposes into water and oxygen gas. calculate the amounts requested if 1.34 moles of dihydrogen dioxide react according to the equation.
you must show all units.
a. moles of oxygen formed
b. moles of water formed
c. mass of water formed
d. mass of oxygen formed

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:30, ayoismeisjjjjuan
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 15:30, lovebaeforlife351
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins. co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 23:30, znewkirk4741
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
Dihydrogen dioxide decomposes into water and oxygen gas. calculate the amounts requested if 1.34 mol...
Questions in other subjects:

Mathematics, 21.03.2020 10:38





Mathematics, 21.03.2020 10:39




Mathematics, 21.03.2020 10:39