Given the reactions below, answer the following questions.
cl_2(g) + f_2(g) rlhar 2clf(g) del...

Chemistry, 25.01.2020 04:31 tsmalls70988
Given the reactions below, answer the following questions.
cl_2(g) + f_2(g) rlhar 2clf(g) delta g degree_rxn = 115.4 kj/mol
cl_2(g) + br_2(g) rlhar 2clbr(g) delta g degree_rxn = -2.0 kj/mol
calculate the delta g degree_rxn for 2clf(g) + br_2(g) rlhar 2clbr(g) + f_2(g) kj/mol

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 11:40, jerrysandoval22
Which of these expressions are correct variations of the combined gas law? p1v1t2 = p2v2t1 both
Answers: 2

Chemistry, 22.06.2019 15:30, ricardotavarez6
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 23.06.2019 02:00, bagofmud8339
The point along a planet's orbit where it is closest to the sun is called the
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 14.11.2020 23:00

Mathematics, 14.11.2020 23:00


Mathematics, 14.11.2020 23:00

English, 14.11.2020 23:00

Biology, 14.11.2020 23:00

Mathematics, 14.11.2020 23:00

Mathematics, 14.11.2020 23:00

Business, 14.11.2020 23:00

Mathematics, 14.11.2020 23:00


 ;
; 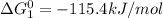
 ;
; 
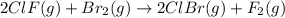 ;
; 


