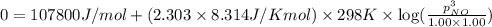Chemistry, 25.01.2020 03:31 6710000831
Consider this reaction occurring at 298 k:
n2o(g) + no2(g) ⇆ 3 no(g)
if a reaction mixture contains only n2o and no2 at partial pressures of 1.0 atm each, the reaction will be spontaneous until some no forms in the mixture.
what maximum partial pressure of no builds up before the reaction ceases to be spontaneous?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, grayfaith16
1) how many electrons are in each energy level of the following elements? a. he b. na c. na d. ne 2) how many valence electrons are percent in the following atoms? a. s b. mg c. be d. cl 3) which of the following elements are stable as atoms? a. he b. o c. cl d. ar if you are able to provide the work as to how you got the answers that would be greatly appreciated. : )
Answers: 1


You know the right answer?
Consider this reaction occurring at 298 k:
n2o(g) + no2(g) ⇆ 3 no(g)
if a...
n2o(g) + no2(g) ⇆ 3 no(g)
if a...
Questions in other subjects:

Computers and Technology, 23.11.2020 03:20


Social Studies, 23.11.2020 03:20



English, 23.11.2020 03:20

Business, 23.11.2020 03:20

Mathematics, 23.11.2020 03:20


Mathematics, 23.11.2020 03:20


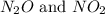 at partial pressures of 1.0 atm each, the reaction will be spontaneous until some NO forms in the mixture.
at partial pressures of 1.0 atm each, the reaction will be spontaneous until some NO forms in the mixture.
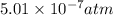
 for above equation follows:
for above equation follows: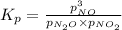
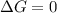 (at equilibrium)
(at equilibrium)
 = Standard Gibbs free energy = 107.8 kJ/mol = 107800 J/mol (Conversion factor: 1 kJ = 1000 J )
= Standard Gibbs free energy = 107.8 kJ/mol = 107800 J/mol (Conversion factor: 1 kJ = 1000 J )