
Chemistry, 25.01.2020 03:31 ceeejay0621
A30-0 g sample of water at 280 k is mixed with 50.0 g of water at 330 k. how would you calculate the final temperature of the mixture assuming no heat is lost to the surroundings?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, notkeandre9
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 20:50, karlyisaunicorn
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2

Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
A30-0 g sample of water at 280 k is mixed with 50.0 g of water at 330 k. how would you calculate the...
Questions in other subjects:


Health, 24.05.2021 20:50

Mathematics, 24.05.2021 20:50



Mathematics, 24.05.2021 20:50




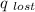 )= heat absorbed by the less hotter sample(
)= heat absorbed by the less hotter sample(  )
)
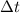 temperature change.
temperature change.


 - 330) = 30 * (
- 330) = 30 * (

