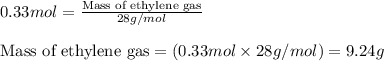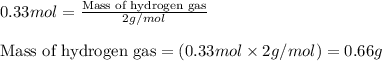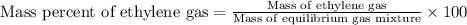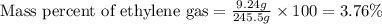Ethylene (ch2ch2) is the starting point for a wide array of industrial chemical syntheses. for example, worldwide about 8.0 x 1010kg of polyethylene are made from ethylene each year, for use in everything from household plumbing to artificial joints. natural sources of ethylene are entirely inadequate to meet world demand, so ethane (ch3ch3) from natural gas is "cracked" in refineries at high temperature in a kineticallycomplex reaction that produces ethylene gas and hydrogen gas. suppose an engineer studying ethane cracking fills a 30.0l reaction tank with 24.0atm of ethane gas and raises the temperature to 800.°c. he believes kp= 0.040 at this temperature. calculate the percent by mass of ethylene the engineer expects to find in the equilibrium gas mixture. round your answer to 2 significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chameleonsarelife
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 09:00, boxergirl2062
Scientific evidence tells us that the cause of earths four season is the tilt of earth as it revolves around the sun. the student is instructed to illustrate this information in a science notebook. how will the student illiterate winter in the northern hemisphere?
Answers: 3

You know the right answer?
Ethylene (ch2ch2) is the starting point for a wide array of industrial chemical syntheses. for examp...
Questions in other subjects:

Mathematics, 30.03.2020 19:23



English, 30.03.2020 19:23



Mathematics, 30.03.2020 19:23



Social Studies, 30.03.2020 19:23

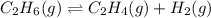
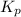 for above equation follows:
for above equation follows: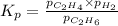
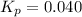
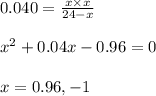
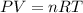 .........(1)
.........(1)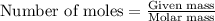 ..........(2)
..........(2)![P=23.04atm\\V=30.0L\\T=800^oC=[800+273]K=1073K\\R=0.0821\text{ L. atm }mol^{-1}K^{-1}](/tpl/images/0469/9843/5217a.png)
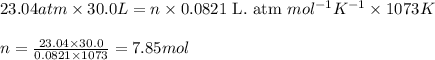
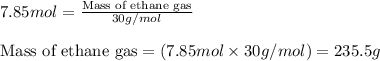
![P=0.96atm\\V=30.0L\\T=800^oC=[800+273]K=1073K\\R=0.0821\text{ L. atm }mol^{-1}K^{-1}](/tpl/images/0469/9843/9eb50.png)