
Chemistry, 25.01.2020 01:31 princeofpowerjr
Awater treatment plant receives the source water with an average ca2+ concentration of 46.9 mg/l and mg2+ concentration of 14.8 mg/l. the plant is treating 80 million gallons of water per day. what mass of solids will be produced per day if all of the calcium and magnesium are converted to caco3(s) and mg(oh)2(s) in the softening process? give your answer in kg.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, montoyaricardo3550
Aroller coaster car is traveling down a track at 22 m/s. the car has a mass of 2000 kg. what is the kinetic energy of the car? a) 22,000 j b) 968,000 j c) 484,000 j d) 44,000 j
Answers: 2

Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 13:00, yaneiryx5476
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:30, Dreynolds1667
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
Awater treatment plant receives the source water with an average ca2+ concentration of 46.9 mg/l and...
Questions in other subjects:


Mathematics, 14.10.2019 16:30

Mathematics, 14.10.2019 16:30



Mathematics, 14.10.2019 16:30


Social Studies, 14.10.2019 16:30


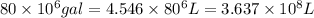
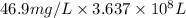



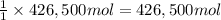 of calcium carbonate
of calcium carbonate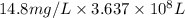
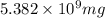
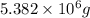
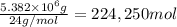
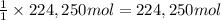 of magnesium hydroxide
of magnesium hydroxide


