Consider the reaction:
2al(s) + fe2o3(s) - al2o3(s) + 2fe(s)
the ah, for fe2o3(s) = -82...

Chemistry, 24.01.2020 23:31 SignoraPenguino
Consider the reaction:
2al(s) + fe2o3(s) - al2o3(s) + 2fe(s)
the ah, for fe2o3(s) = -824.3 kj/mole. the ah, for al2o3(s) = -1675.7 kj/mole.
finish the equation.
ahxn = [(1)
c
y
kj/mole) + (2)
y kj/mole)] - [(1)
kj/mole) + (2) (
kj/mole)]

Answers: 2


Other questions on the subject: Chemistry



You know the right answer?
Questions in other subjects:

Arts, 04.05.2021 14:40

Biology, 04.05.2021 14:40

Biology, 04.05.2021 14:40

Computers and Technology, 04.05.2021 14:40

English, 04.05.2021 14:40


Mathematics, 04.05.2021 14:40


English, 04.05.2021 14:40

Mathematics, 04.05.2021 14:40


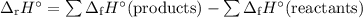
![\begin{array}{rcl}\Delta_{\text{r}}H^{\circ} & = & [1(-1675.7) + 2(0)] - [2(0) - 1(-824.3)]\\& = & -1675.7 + 824.3\\& = & \textbf{-851.4 kJ/mol}\\\end{array}\\\text{The enthalpy change is } \large \boxed{\textbf{-851.4 kJ/mol}}](/tpl/images/0469/5682/d5fae.png)


