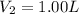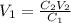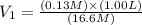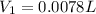Chemistry, 24.01.2020 21:31 RoyalGurl01
You wish to prepare 0.13 m hno3 from a stock solution of nitric acid that is 16.6 m. how many milliliters of the stock solution do you require to make up 1.00 l of 0.13 m hno3?

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, marcusajns
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
You wish to prepare 0.13 m hno3 from a stock solution of nitric acid that is 16.6 m. how many millil...
Questions in other subjects:

Mathematics, 12.01.2021 23:20


Mathematics, 12.01.2021 23:20


Physics, 12.01.2021 23:20


Geography, 12.01.2021 23:20

Computers and Technology, 12.01.2021 23:20

Mathematics, 12.01.2021 23:20

Mathematics, 12.01.2021 23:20

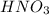
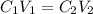
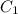 and
and 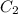 are initial and final concentration respectively.
are initial and final concentration respectively. 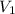 and
and 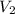 are initial and final volume respectively.
are initial and final volume respectively.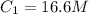 ,
, 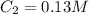 and
and