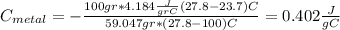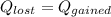For the following scenarios what is the metal? a piece of metal weighing 59.047 g was heated to 100.0 degree c and then put it into 100.0 ml of water (initially at 23.7 degree c). the metal and water were allowed to come to an equilibrium temperature, determined to be 27.8 degree c. assuming no heat lost to the environment, calculate the specific heat of the metal. a 25.6 g piece of metal was taken from a beaker of boiling water at 100.0 degree c and placed directly into a calorimeter holding 100.0 ml of water at 25.0 degree c. the calorimeter heat capacity is 1.23 j/k. given that the final temperature at thermal equilibrium is 26.2 degree c, determine the specific heat capacity of the metal.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:30, darrriannn7241
What is the correct lewis structure for chloroform chcl3
Answers: 1

Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1
You know the right answer?
For the following scenarios what is the metal? a piece of metal weighing 59.047 g was heated to 100...
Questions in other subjects:


Mathematics, 01.06.2021 20:10




Mathematics, 01.06.2021 20:10

Biology, 01.06.2021 20:10

History, 01.06.2021 20:10

History, 01.06.2021 20:10

Mathematics, 01.06.2021 20:10



 the mass of the metal
the mass of the metal Is the value that we need to find
Is the value that we need to find represent the final temperature of equilibrium for the metal and the water
represent the final temperature of equilibrium for the metal and the water represent the initial temperature for the metal
represent the initial temperature for the metal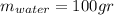 since the density is 1g/ml
since the density is 1g/ml the specific heat for the liquid water
the specific heat for the liquid water the initial temperature for the water
the initial temperature for the water if we have balance then we have this:
if we have balance then we have this:

 we got:
we got: