
Chemistry, 24.01.2020 18:31 4300402428
Read the following descriptions of the element zinc and indicate which are physical properties and which are chemical properties. drag the items into the appropriate bins. reset zinc has a hardness on the mohs scale of 2.5 and a density of 7.13 g/cm3 at 25 c. zinc melts at 420 c. when zinc granules are added to dilute sulfuric acid, hydrogen is given off and the metal dissolves. it reacts slowly with oxygen gas at elevated temperatures to form zinc oxide, zno. physical properties chemical properties

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, cutebab4786
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 11:50, trinityrae4657
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:30, emmalybrown
A50.0 ml sample of gas at 20.0 atm of pressure is compressed to 40.0 atm of pressure at constant temperature. what is the new volume? 0.0100 ml 0.325 ml 25.0 ml 100. ml
Answers: 1
You know the right answer?
Read the following descriptions of the element zinc and indicate which are physical properties and w...
Questions in other subjects:

Mathematics, 03.12.2021 19:50


Mathematics, 03.12.2021 19:50





Computers and Technology, 03.12.2021 19:50

Social Studies, 03.12.2021 19:50

 .Zinc melts at
.Zinc melts at 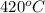 .
.

