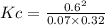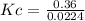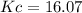Chemistry, 24.01.2020 10:31 jeffhuffle17
Areaction mixture that consisted of 0.35 molh2and 1.6 mol i2 was introduced into a 2 l flask and heated. at the equilibrium, 60% of the hydrogen gas had reacted. what is the equilibrium constant kc for the reactionh2(g) + i2(g)⇀↽2 hi(g)at this temperature?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, awdadaddda
How air particles exert a pressure on the inside of the balloon
Answers: 1


Chemistry, 22.06.2019 13:30, amandajbrewerdavis
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Areaction mixture that consisted of 0.35 molh2and 1.6 mol i2 was introduced into a 2 l flask and hea...
Questions in other subjects:




Mathematics, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

Mathematics, 12.12.2020 17:00

Spanish, 12.12.2020 17:00


Mathematics, 12.12.2020 17:00

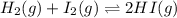
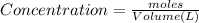
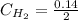
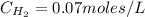
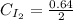
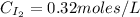
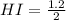
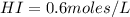
![Kc=\frac{[HI]^{2}}{[H_{2}][I_{2}]}](/tpl/images/0468/7785/b83f2.png)